In collaboration with Genomis Ltd UK
Progenomis®: genomics for the health of your future child


The Progenomis® couple screen analyzes, through massive parallel sequencing (Next Generation Sequencing – NGS), the DNA of more than 670 genes in both reproductive partners. These genes are involved in several hundreds of severe recessive genetic diseases occurring with a combined frequency of at least 1 in 200 to 1 in 300 births (see list of genes and diseases). The current list of genes and the associated disorders may be expanded in the future.
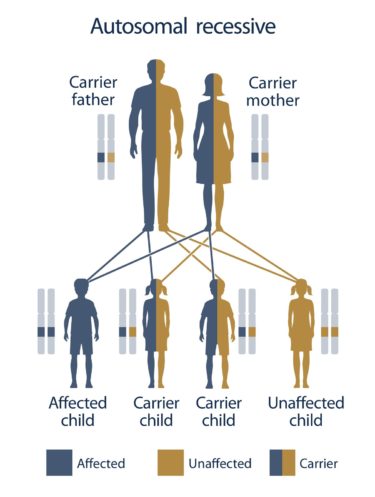
A positive test result for both partners in any single gene gives valuable information about your reproductive risks and prevents the birth of an affected child.
In case both partners are carriers of known pathogenic mutations or new obligatory pathogenic mutations in any one of the 670 genes, or if the mother is a carrier of an X-linked gene mutation, the test provides valuable information regarding the reproductive risks of the parents for having an affected child.
It is easily understood that prior knowledge of the existence of mutations in the same gene in both prospective parents offers the possibility of personalized reproductive measures, such as prenatal diagnosis or preimplantation genetic diagnosis through IVF, thus preventing the birth of an affected child.
Targeted carrier testing in the population for single-gene disorders is not something new. For example, carrier testing for cystic fibrosis (incidence ~1 in 2,500 births) has been a common practice in most countries-populations, while carrier testing for Tay-Sach’s disease, Canavan disease, Nieman Pick Disease Type A, Bloom syndrome and Gaucher’s disease is also common among individuals of Ashkenazi Jewish descent.
Typically, carriers for any of the ~670 severe recessive diseases included in the Progenomis® pan-ethnic couple screen are asymptomatic and may present without any prior family history of the disorder. Therefore, they cannot be identified by any other way and this is the main reason why children affected by these genetic diseases are born and diagnosed after birth.
PROGENOMIS GENES AND DISEASE LIST ENGLISH.pdf
The Progenomis® test, if widely applied in the future in various populations, will decisively contribute to reducing the birth of severely affected children with recessive or X-linked inherited diseases.
Technical details
Testing requires a biological sample (blood or saliva) from both reproductive partners (a sample collection and shipment kit will be provided). Prior to testing, both individuals must complete and sign the Test Information and Consent Form, with the option of receiving online pre-test genetic counseling.
The samples are shipped to our laboratory where genomic DNA is extracted, followed by massive parallel sequencing (NGS) of the ~670 genes. Sequence reads are then aligned and mapped to the human genome reference sequence and all variants and putative mutations are prioritized through a proprietary specially developed in-house bioinformatics pipeline.
Only known pathogenic or obligatory pathogenic (nonsense, frameshift, consensus splice-site) mutations will be reported, together with a full clinical assessment and clinical genetic evaluation.
The test also includes MLPA analysis for the detection of deletions/duplications of the SMN1 and DMD genes, associated with the genetic disorders Spinal Muscular Atrophy ‐ SMA (OMIM 253300) and Duchenne/Becker muscular dystrophy (DMD/BMD ‐ OMIM 310200). In females only, we also include carrier testing for Fragile X syndrome.
A final clinical report is released, containing all the relevant findings and recommendations for further actions, where applicable. Positive findings and reproductive options are discussed in the context of a personalized genetic counseling session.
Test limitations
Results are evaluated and reported based on current knowledge.
The sensitivity of the Progenomis® test is ~95% and it will not detect numerical or structural chromosomal abnormalities as well as deletions or duplications >15-20 base-pairs.
A negative result does not exclude totally the presence of a pathogenic mutation in the offspring, which may result from rare events such as, for example, a de novo mutation or the presence of germline mosaicism for a mutation in a parent.
InterGenetics is a Ion Torrent™ Certified Service Provider for Ion AmpliSeq sequencing on the Ion Proton platform.
The test is executed in 3-4 weeks
InterGenetics is a Ion Torrent™ Certified Service Provider for Ion AmpliSeq sequencing on the Ion Proton platform.
