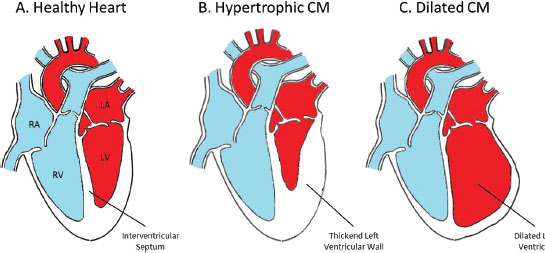
Cardiomyopathies, or heart muscle diseases, are a common cause of heart failure, which is a leading cause of death in most societies. There are several different types of cardiomyopathy, many of which can have a hereditary cause. The two major types of cardiomyopathy are dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM). Many different genetic causes of cardiomyopathy have been discovered and identifying the underlying genetic cause can influence medical management decisions and preventive screening options for at-risk relatives. Careful consideration of the personal and family history should be given when deciding which cardiomyopathy panel is most appropriate for testing in an individual patient or family.
Determining the genetic cause of cardiomyopathies can be complex. There have been many different genes and many different mutations involved in inherited predisposition to cardiomyopathy. The genetic cause is further complicated because there is significant phenotypic overlap between the different types of cardiomyopathy and also significant overlap in the genetic causes between subtypes. For example, at least 19 genes have been implicated in both HCM and DCM, and occasionally a person who initially had HCM, may not be diagnosed with heart disease until the disease is very advanced and presents as DCM. In addition, different types of cardiomyopathy can run in families. Some families present with exclusively one type of cardiopmyopathy, such as HCM, whereas in another family we might see the same genetic alteration causing different types of cardiomyopathy in different family members, such as DCM and HCM. Due to this overlap in genetic causes and in the symptoms and presentation of disease for different types of cardiomyopathy, a clinician may consider testing for genes associated with multiple subtypes of cardiomyopathy in one comprehensive test.