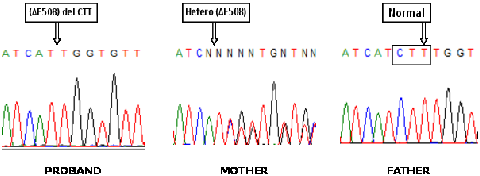
This test calculates the probability of a fetus with Down syndrome (trisomy 21) and Edwards syndrome (trisomy 18), taking into account the age of the pregnant woman, the levels of certain biochemical markers produced during pregnancy and detected in maternal blood, with the aid of a special software.
The test is applied utilizing maternal dry blood samples and marker levels are determined by automated ELISA techniques
During the 1st trimester (10th – 13th week): measurement of the levels of β-hCG (free beta-chorionic gonadotrophin) and placental protein PAPPa. A statistical extrapolation of the risk to the fetus is then obtained, through specialized computer software, combining biochemical marker levels and the provided measurement of the nuchal translucency (NT) of the fetus. The test will also indicate the risk for trisomy 18 (Edwards syndrome).
During the 2nd trimester (16th – 22nd week): measurement of the levels of β-hCG, α-FP (alpha-fetoprotein) and uE3 (unconjugated estriol), again from maternal dry blood samples and processed as above. Also, as above, the risk for trisomy 18 (Edwards syndrome) is included.
The results are always presented in the form of probability and the high-risk limit (cut-off) for a pregnancy with Down syndrome is 1/250. Above this threshold (e.g. a probability of 1/200 or 1/100 etc.), the pregnancy is characterized as high-risk and prenatal chromosomal testing is then recommended by obtaining chorionic villi (in the 1st trimester) or amniotic fluid (2nd trimester), which are both fully diagnostic.
From our longstanding experience, it is estimated that ~4-5% of cases examined in the 1st trimester and ~6% in the 2nd trimester will yield a high risk result. However, in cases of low-risk pregnancies, the birth of a child with Down syndrome or with other chromosomal abnormalities cannot be completely excluded, as the test is able to detect: about 87% of Down syndrome pregnancies the 1st trimester, with the inclusion of NT(~62% without NT), and ~68% of Down syndrome pregnancies the 2nd trimester.